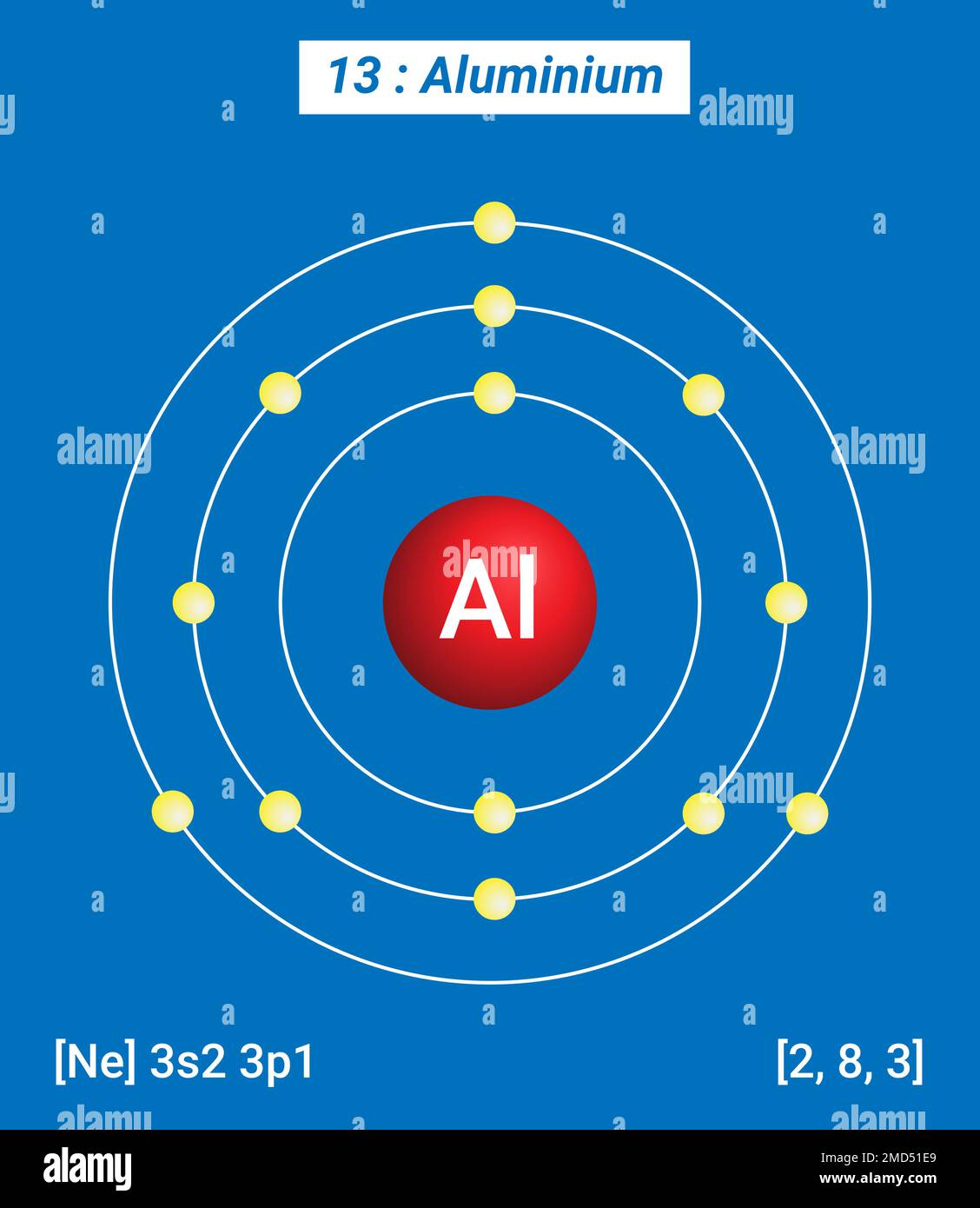
Aluminum Atoms To Grams . use atoms to grams calculator to get accurate, quick, and easy atom to grams conversions. this chemistry video tutorial explains the conversion process of atoms to. one mole of al atoms has a mass in grams that is numerically equivalent to the atomic mass of aluminum. The number of particles in one mol of a substance (given by avogadro's number) and the molar mass of the substance. With these two quantities you can find the number of grams of the substance that contain a given number of atoms. calculate the molar mass of aluminum in grams per mole or search for a chemical formula or substance. to convert from atoms to grams you will need two conversion factors: the compound’s formula shows that each molecule contains seven carbon atoms, and so the number of c atoms in the provided. Aluminium element mass percent aluminium/aluminum 26.9815g aluminium/aluminum 26.9815g.
from www.alamy.com
this chemistry video tutorial explains the conversion process of atoms to. calculate the molar mass of aluminum in grams per mole or search for a chemical formula or substance. Aluminium element mass percent aluminium/aluminum 26.9815g aluminium/aluminum 26.9815g. The number of particles in one mol of a substance (given by avogadro's number) and the molar mass of the substance. With these two quantities you can find the number of grams of the substance that contain a given number of atoms. one mole of al atoms has a mass in grams that is numerically equivalent to the atomic mass of aluminum. the compound’s formula shows that each molecule contains seven carbon atoms, and so the number of c atoms in the provided. to convert from atoms to grams you will need two conversion factors: use atoms to grams calculator to get accurate, quick, and easy atom to grams conversions.
Atom model aluminium Stock Vector Images Alamy
Aluminum Atoms To Grams this chemistry video tutorial explains the conversion process of atoms to. The number of particles in one mol of a substance (given by avogadro's number) and the molar mass of the substance. to convert from atoms to grams you will need two conversion factors: calculate the molar mass of aluminum in grams per mole or search for a chemical formula or substance. Aluminium element mass percent aluminium/aluminum 26.9815g aluminium/aluminum 26.9815g. one mole of al atoms has a mass in grams that is numerically equivalent to the atomic mass of aluminum. this chemistry video tutorial explains the conversion process of atoms to. use atoms to grams calculator to get accurate, quick, and easy atom to grams conversions. With these two quantities you can find the number of grams of the substance that contain a given number of atoms. the compound’s formula shows that each molecule contains seven carbon atoms, and so the number of c atoms in the provided.
From www.toppr.com
Calculate the number of aluminium ions present in 0.051 g of aluminium Aluminum Atoms To Grams use atoms to grams calculator to get accurate, quick, and easy atom to grams conversions. The number of particles in one mol of a substance (given by avogadro's number) and the molar mass of the substance. Aluminium element mass percent aluminium/aluminum 26.9815g aluminium/aluminum 26.9815g. With these two quantities you can find the number of grams of the substance that. Aluminum Atoms To Grams.
From www.youtube.com
grams to atoms calculations YouTube Aluminum Atoms To Grams this chemistry video tutorial explains the conversion process of atoms to. one mole of al atoms has a mass in grams that is numerically equivalent to the atomic mass of aluminum. to convert from atoms to grams you will need two conversion factors: Aluminium element mass percent aluminium/aluminum 26.9815g aluminium/aluminum 26.9815g. With these two quantities you can. Aluminum Atoms To Grams.
From brainly.in
draw the geometric representation of aluminium atom Brainly.in Aluminum Atoms To Grams the compound’s formula shows that each molecule contains seven carbon atoms, and so the number of c atoms in the provided. The number of particles in one mol of a substance (given by avogadro's number) and the molar mass of the substance. this chemistry video tutorial explains the conversion process of atoms to. Aluminium element mass percent aluminium/aluminum. Aluminum Atoms To Grams.
From ar.inspiredpencil.com
Atomic Structure Of Aluminum Aluminum Atoms To Grams use atoms to grams calculator to get accurate, quick, and easy atom to grams conversions. the compound’s formula shows that each molecule contains seven carbon atoms, and so the number of c atoms in the provided. With these two quantities you can find the number of grams of the substance that contain a given number of atoms. . Aluminum Atoms To Grams.
From stock.adobe.com
Bohr model diagram of Aluminium Al in atomic physics Stock Vector Aluminum Atoms To Grams The number of particles in one mol of a substance (given by avogadro's number) and the molar mass of the substance. With these two quantities you can find the number of grams of the substance that contain a given number of atoms. to convert from atoms to grams you will need two conversion factors: one mole of al. Aluminum Atoms To Grams.
From www.vrogue.co
Aluminum Atom Diagram vrogue.co Aluminum Atoms To Grams With these two quantities you can find the number of grams of the substance that contain a given number of atoms. calculate the molar mass of aluminum in grams per mole or search for a chemical formula or substance. this chemistry video tutorial explains the conversion process of atoms to. The number of particles in one mol of. Aluminum Atoms To Grams.
From circuitwiringgear.z21..core.windows.net
Aluminum Electron Dot Diagram Aluminum Atoms To Grams Aluminium element mass percent aluminium/aluminum 26.9815g aluminium/aluminum 26.9815g. to convert from atoms to grams you will need two conversion factors: calculate the molar mass of aluminum in grams per mole or search for a chemical formula or substance. the compound’s formula shows that each molecule contains seven carbon atoms, and so the number of c atoms in. Aluminum Atoms To Grams.
From www.chegg.com
Solved How many aluminum atoms are there in 3.50 grams of Aluminum Atoms To Grams With these two quantities you can find the number of grams of the substance that contain a given number of atoms. one mole of al atoms has a mass in grams that is numerically equivalent to the atomic mass of aluminum. to convert from atoms to grams you will need two conversion factors: this chemistry video tutorial. Aluminum Atoms To Grams.
From www.alamy.com
Atom model aluminium Stock Vector Images Alamy Aluminum Atoms To Grams With these two quantities you can find the number of grams of the substance that contain a given number of atoms. this chemistry video tutorial explains the conversion process of atoms to. use atoms to grams calculator to get accurate, quick, and easy atom to grams conversions. Aluminium element mass percent aluminium/aluminum 26.9815g aluminium/aluminum 26.9815g. one mole. Aluminum Atoms To Grams.
From brainly.in
Atomic structure of aluminum Brainly.in Aluminum Atoms To Grams to convert from atoms to grams you will need two conversion factors: calculate the molar mass of aluminum in grams per mole or search for a chemical formula or substance. the compound’s formula shows that each molecule contains seven carbon atoms, and so the number of c atoms in the provided. Aluminium element mass percent aluminium/aluminum 26.9815g. Aluminum Atoms To Grams.
From www.dreamstime.com
Aluminum Atom, with Mass and Energy Levels. Stock Vector Illustration Aluminum Atoms To Grams calculate the molar mass of aluminum in grams per mole or search for a chemical formula or substance. The number of particles in one mol of a substance (given by avogadro's number) and the molar mass of the substance. one mole of al atoms has a mass in grams that is numerically equivalent to the atomic mass of. Aluminum Atoms To Grams.
From www.answersarena.com
[Solved] Calculate the number of grams of aluminum that wi Aluminum Atoms To Grams The number of particles in one mol of a substance (given by avogadro's number) and the molar mass of the substance. the compound’s formula shows that each molecule contains seven carbon atoms, and so the number of c atoms in the provided. calculate the molar mass of aluminum in grams per mole or search for a chemical formula. Aluminum Atoms To Grams.
From www.alamy.com
Aluminum atom, with mass and energy levels. Vector illustration Stock Aluminum Atoms To Grams this chemistry video tutorial explains the conversion process of atoms to. use atoms to grams calculator to get accurate, quick, and easy atom to grams conversions. calculate the molar mass of aluminum in grams per mole or search for a chemical formula or substance. Aluminium element mass percent aluminium/aluminum 26.9815g aluminium/aluminum 26.9815g. to convert from atoms. Aluminum Atoms To Grams.
From www.youtube.com
How to Convert Grams Al to Moles (and Moles Al to Atoms) YouTube Aluminum Atoms To Grams use atoms to grams calculator to get accurate, quick, and easy atom to grams conversions. to convert from atoms to grams you will need two conversion factors: Aluminium element mass percent aluminium/aluminum 26.9815g aluminium/aluminum 26.9815g. The number of particles in one mol of a substance (given by avogadro's number) and the molar mass of the substance. one. Aluminum Atoms To Grams.
From sites.google.com
Aluminum Table of Elements by Shrenil Sharma Aluminum Atoms To Grams one mole of al atoms has a mass in grams that is numerically equivalent to the atomic mass of aluminum. calculate the molar mass of aluminum in grams per mole or search for a chemical formula or substance. this chemistry video tutorial explains the conversion process of atoms to. Aluminium element mass percent aluminium/aluminum 26.9815g aluminium/aluminum 26.9815g.. Aluminum Atoms To Grams.
From www.museoinclusivo.com
How Many Aluminum Atoms Are in 3.78 Grams of Aluminum? Aluminum Aluminum Atoms To Grams this chemistry video tutorial explains the conversion process of atoms to. With these two quantities you can find the number of grams of the substance that contain a given number of atoms. the compound’s formula shows that each molecule contains seven carbon atoms, and so the number of c atoms in the provided. Aluminium element mass percent aluminium/aluminum. Aluminum Atoms To Grams.
From steamever.weebly.com
Conversion Of Atoms To Grams steamever Aluminum Atoms To Grams this chemistry video tutorial explains the conversion process of atoms to. With these two quantities you can find the number of grams of the substance that contain a given number of atoms. the compound’s formula shows that each molecule contains seven carbon atoms, and so the number of c atoms in the provided. calculate the molar mass. Aluminum Atoms To Grams.
From www.museoinclusivo.com
How Many Aluminum Atoms Are in 3.78 Grams of Aluminum? Aluminum Aluminum Atoms To Grams one mole of al atoms has a mass in grams that is numerically equivalent to the atomic mass of aluminum. Aluminium element mass percent aluminium/aluminum 26.9815g aluminium/aluminum 26.9815g. The number of particles in one mol of a substance (given by avogadro's number) and the molar mass of the substance. the compound’s formula shows that each molecule contains seven. Aluminum Atoms To Grams.